If you’ve spent anytime outside hiking, you’ve probably encountered a passing rain shower. While this is typically a minor inconvenience for most of us, the presence of rain is vital for the survival of all life on Earth. Water is essential for the survival of all forms of life. Without water, land animals could not stay hydrated, all aquatic life would go extinct, and all plant life would cease to exist. But, what if I told you this water that’s vital for life, is slowly damaging the world around us.

THE WATER CYCLE
In order to understand acid rain, and rain in general, we first need to learn about the water cycle. The water cycle involves a continuous cycle of water between the earth’s surface and earth’s atmosphere. The water cycle has 4 main phases; evaporation, transpiration, condensation and precipitation.
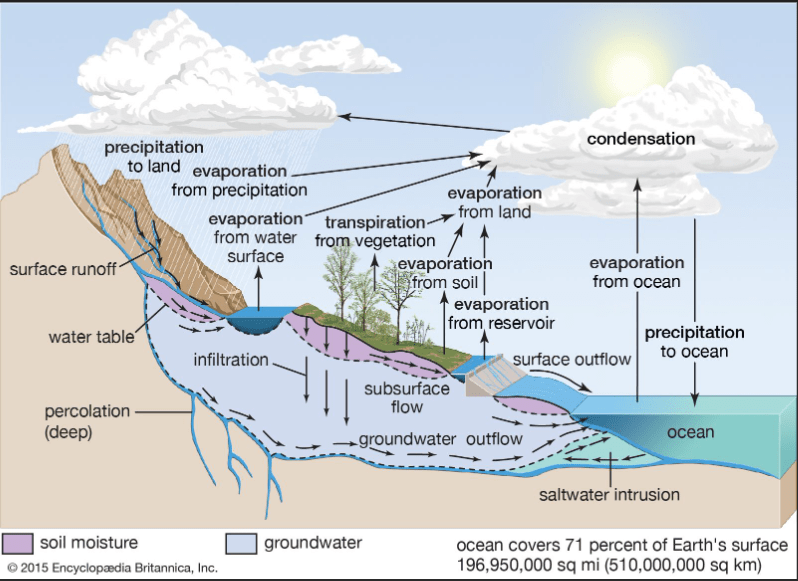
Evaporation is when water in a liquid state becomes water vapor, which is water in a gaseous state. The largest source of water for evaporation are the oceans. Transpiration is where water evaporates from the leaves of plants and other vegetation. Evaporation from all surfaces or is called total evaporation or evapotranspiration.
Condensation occurs when gaseous water transforms back into a liquid state. Condensation is responsible for the formation of clouds (to learn more about clouds and their formation, read my other blog post here!). Clouds will contain the liquid water until the atmosphere can no longer support the moisture level. If you wear glasses, then you likely experienced this phenomena on a small scale. You’ve experienced this effect if you ever spent time outside on a humid day, then walked into an air conditioned room. The fog on your glasses is condensation; water vapor turning to liquid.
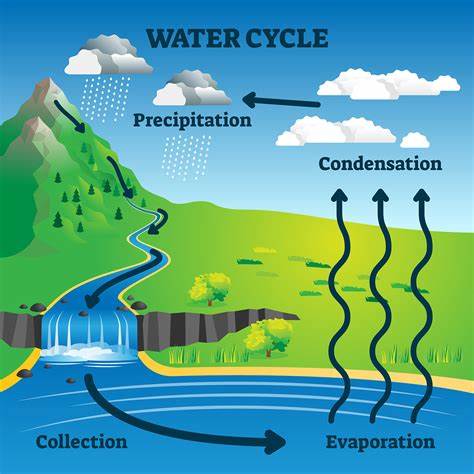
The last phase of the water cycle is precipitation. Wind and air currents in the atmosphere push the clouds inland. Precipitation is when the liquid water falls from the atmosphere to the surface in the form of rain, snow or hail. As rain precipitates over land, liquid water runs into the ground, rivers, lakes and other larger bodies of water. This deposition of liquid water is called runoff. Snow melt is also a vital source of runoff in the winter as new streams and pools are formed.
THE ATMOSPHERE
Since water vapor resides in the atmosphere, it is no surprise that other chemicals in the atmosphere can affect the water that precipitates. Our atmosphere, and the air we breathe, is about 78% nitrogen and 20.9% oxygen. The remining 2% is made up of various chemicals and elements from the periodic table. It is this 2% that make up the ingredients needed to form acid rain.
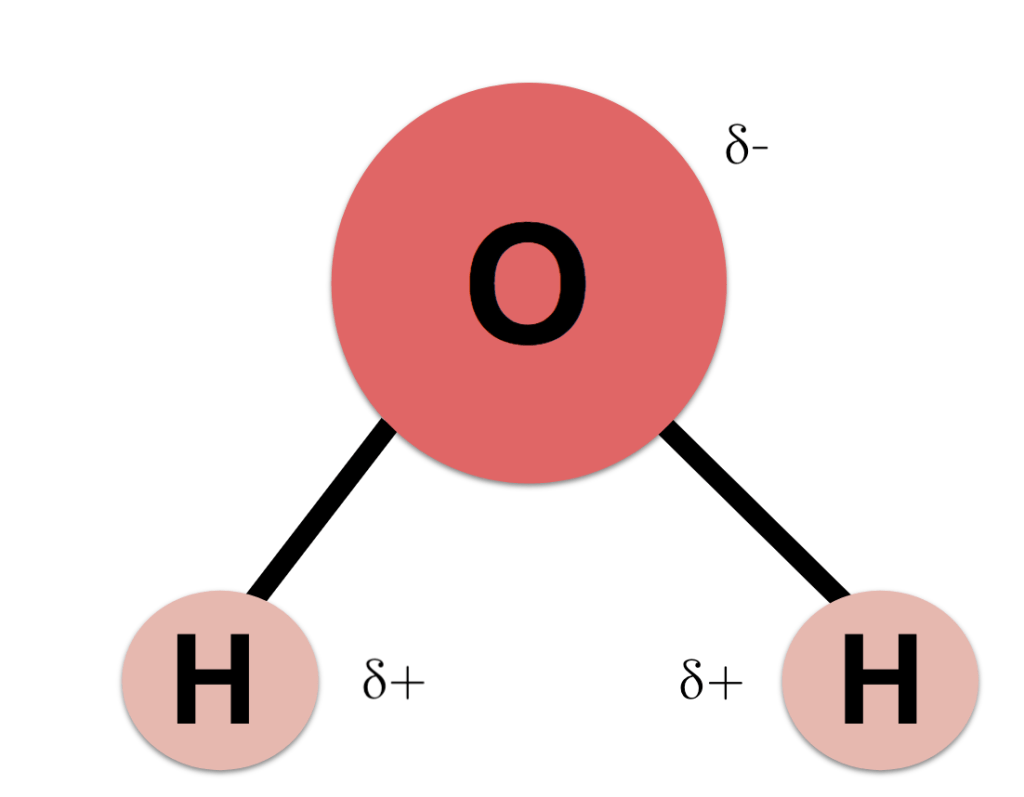
Liquid water is formed by joining one oxygen atom and two hydrogen atoms. Water is a what chemists call a polar molecule. This means the molecule has a charge to it. Simply put, the number of protons and electrons are not evenly shared. As a result, the hydrogen side of the molecule has a slightly positive charge, while the oxygen has a slightly negative charge. This polarity means the water molecule can easily bond with other molecules.
Our atmosphere is full of molecules including sulfur oxides (SOx) and nitrogen oxides (NOx). For reference, the “X” denotes another atom or atoms present in the molecule. NOx and SOx molecules can represent a wide variety of products. These sulfur and nitrogen compounds mix with the water to form H2SO4– (sulfuric acid) and HNO3– (nitric acid). Water can also absorb carbon dioxide, forming carbonic acid (H2CO3–).
Now this doesn’t mean acid is falling from the sky when it rains. The concentrations of these acids are extremely low. However, they are present, and the effect is severe enough to change the pH of our lakes, oceans, and affect our ecosystem.
WHAT IS PH?
In chemistry, scientists can determine the number of hydrogen atoms present in a mixture. This number is represented in a scale ranging from 0-14 with 0 being very concentrated with hydrogen and containing very little OH (Hydroxide) molecules. A pH of 14 indicates a high presence of hydroxide molecules and very few hydrogen atoms. A solution or mixture with a pH of 7 indicates an equal balance of hydrogen atoms and hydroxide molecules.
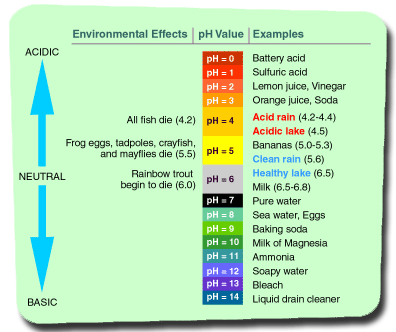
Normal rain has a pH of about 5.6 (in controlled environments, water can have a pH of 7). Acid rain is defined as having a pH of 4.5 or less. To put pH in perspective, orange juice has a pH of about 3, while laundry detergent has a pH of about 14. With NOx and SOx compounds being directly linked to increased hydrogen in the water cycle, it should come as no surprise that countries have began to pass laws regulating sulfur and nitrogen emissions. However, preventing the formation of these acids is more challenging than one may think.
SOURCES OF ACID RAIN
NOx and SOx compounds can come from a variety of natural sources such as volcanic eruptions, wildfires and lightening strikes. There are also man made sources of NOx and SOx molecules such as burning of fossil fuels and emissions. According to an article published in Science Direct, the electric power industry is the largest contributor to SOx and NOx compounds. One estimate has the electric industry accounting for two-thirds of SOx compounds and one quarter of NOx compounds.
Not even renewal energy sources such as solar and wind generation are free from SOx and NOx production. Metals such as Lithium and Cadmium are essential for batteries and solar panels. The only way to extract these metals from the Earth is through mining operations, which produce a lot of NOx and SOx emissions. Recycling these products after their lifespan produces a lot of harmful pollution and both lithium and cadmium are highly toxic. Nearly every industry can be connected to a process that affects or directly creates acid rain.
THE EFFECTS OF ACID RAIN
Acid rain can have major detrimental effects to the environment. A small change in pH can cause some species to become extinct. The most obvious effects will occur in the oceans, lakes and rivers. A pH of 5 inhibits the reproductive process of some species of fish and other aquatic animals. Coral reefs can begin to die, leaving aquatic animals without food or shelter. As mass die offs occur, toxins from the decaying vegetation and aquatic life are released into the water, which will eventually affect all aquatic life.
Species living on land will also be affected. Acid rain will dissolve nutrients such as potassium and magnesium in the soil that plants and trees need to survive. With the reduction in essential nurturance, atoms of Aluminum and other harmful elements will reach toxic levels. With reduced resources in the soil, trees and vegetation will become scarce. The lack of vegetation means there’s less food for animals like bears and wolfs. The change in soil composition also means farmers will have a harder time growing crops.

Even dry environments that don’t see a lot of rain will suffer. NOx and SOx emitted in the air can travel between nations. Gaseous acidic particles not contained in rain or clouds can move through the air currents and settle on areas far from where they originated. If inhaled, these gases could lead to heart and lung problems in any air breathing lifeform. Man made metal and stone structures will also suffer damage. The Roman Colosseum, Westminster Abby, Cologne Cathedral and other structures world wide have suffered damage directly related to acid rain.
WHAT CAN WE DO TO STOP ACID RAIN
Acid rain is a world wide problem. For example, acidification of lakes in Scandinavia has been linked to emissions from the United Kingdom. With this problem being so broad, it may be overwhelming when we try and stop it. The biggest impact in preventing acid rain is with legislation. The United States enacted the Clean Air Act in 1963 and has been amended many times including 1970 which added ambient air standards and in 1990 to further reduce levels of NOx and SOx. Many developed nations have enacted similar laws.
Most nations also have vehicle and transportation emission standards. This is the reason why we need to have our vehicles emissions tested fairly regularly. In our homes, we can switch to more energy efficient lightbulbs. Newer lights use less electricity, thus reducing the NOx and SOx from the electric grid. The use of low sulfur gas and coal will also have a major impact on acid rain around the world.

No matter the circumstances surrounding emissions regulations, all of us can agree that something needs to be done. Humans are using up the resources on planet Earth faster than nature can replenish them. If we keep emitting at the rate we currently are, we won’t have a planet to call home.
REFERENCES
- https://www.britannica.com/science/water-cycle
- https://www.usgs.gov/special-topics/water-science-school/science/condensation-and-water-cycle
- https://education.nationalgeographic.org/resource/hydrologic-cycle/
- https://www.noaa.gov/jetstream/atmosphere
- https://www.epa.gov/acidrain/what-acid-rain
- https://www.usgs.gov/media/images/ph-scale
- https://www.sciencedirect.com/topics/chemistry/acid-precipitation
- https://www.usgs.gov/mission-areas/water-resources/science/acid-rain
- https://www.boem.gov/air-quality-act-1967-or-clean-air-act-caa
- https://www.britannica.com/science/acid-rain/History
